Redox Titration Practical Answers First Year
Chemistry practical answers
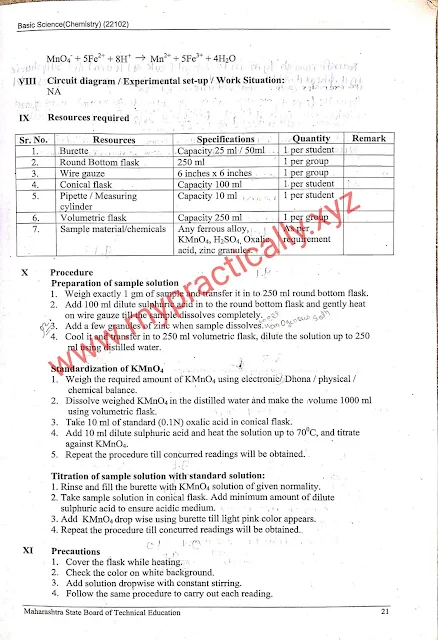
Experiment number 3 Redox titration
Hii friends in this article you will get rid of reaction titration Practical answers
Hello students in this practical you will get practical answers related to Redox titration or redox reaction
Now we will how this practical redox titration practical and the search will help you to acquire basic knowledge related to chemistry
First of all reduction and oxidation are one of the most and significant phenomena in the chemistry industry actually the chemical process is done in chemical industries the chemical reaction and property identification of different metals and their alloys. The oxidation-reduction process jointly leads towards the redox titration redox reaction which helps in the determination of iron content in a given sample solution using redox titration. This will help students for diploma training to understand the metallic property of a metal that can be used in solving Tum broad-based engineering problems and their solutions.
 |
| Redox Titration Practical Answers First-Year Chemistry practical answers |
What is the minimum theoretical background related to redox titration practical
Characteristics of metal lead towards oxidation-reduction phenomenon as we all know. Oxidation is basically a process of medicine of Oxygen and removal of hydrogen or electron and reduction in the process of addition of hydrogen electrons or removal of oxygen
In simple words, oxidation is a process in which edition of oxygen is done and removal of hydrogen is taken out and reduction is vice versa of the oxidation process
Oxidizing agents and ask that gain one or more electrons and get reduced. Are substance that one or more electrons and get oxidized. Are electron acceptors and reducing agents are electron donors.
A solution whose concentration is known is called as standard solutions full stop the substance used to prepare a standard solution is called as primary standard that is oxalic acid and sodium carbonate are some examples of the primary standard.
Relation based oxidation and reduction reaction between titrate and analysed is called redox titration.
The redox titration method can be used to determine the strength of the reduction oxidant using a Redox indicator.
Page 1
Page 2
Page 3
Page 4
Page 5
This video will help you for getting science practical and search redox titration experiment number three basic Science 1st year
Hope this article will help you for preparation of Msbte lab manual doubt please contact in comment section Thank You
- Related Searches:-
- Redox Titration Practical Answers
- "redox titration with potassium permanganate lab report"
- "determine the molarity of given kmno4 solution with the help of supplied m20 mohrs salt solution"
- "determine the strength of kmno4 solution by the given m by 10 oxalic acid solution"
- "determine the strength of kmno4 solution with the help of n10 oxalic acid"
- "determine the strength of kmno4 solution with the help of standard oxalic acid solution"
- "what indicator is used in the titration of oxalic acid with kmno4"
- "determine the strength of kmno4 solution with the help of m10 oxalic acid"
- "determine the molarity of given kmno4 solution by titrating it against m20 oxalic acid solution"





it is Rong
ReplyDelete